产品详情

- 产品/服务:PQ/Porapak Q填充柱 医疗保健产品灭菌环氧乙烷残留测定
- 型 号:PQ/Porapak Q填充柱
- 品 牌:浩瀚色谱
- 单 价:面议
- 更新日期:2022-05-03
- 有效期至:长期有效
- 浏览次数:1234
- 立即询价
产品简介
医疗保健产品灭菌环氧乙烷残留测定,芝麻香白酒,3-甲硫基丙醇,室内空气,焦炉煤气,炼厂气,天然气,,多氯联苯,植物油,增塑剂,塑化剂,医疗器械,过氧化物,氨水,氨气...
产品详细介绍
医疗保健产品灭菌环氧乙烷残留测定
医疗保健产品灭菌环氧乙烷残留测定 详细信息:
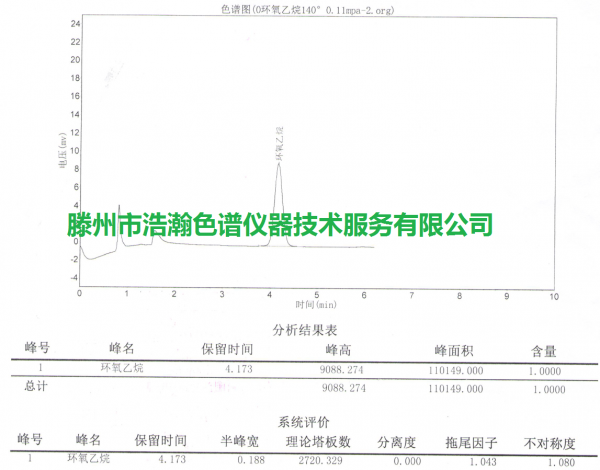
浩瀚色谱(山东)应用技术开发有限公司建立气相色谱法检测口罩、防护服等医疗防护用品中环氧乙烷的残留量,为生产厂家改进灭菌工艺提供数据支持。气相色谱法采用顶空进样、FID检测器,环氧乙烷的质量浓度在0.5~8 μg/mL范围内与色谱峰面积线性关系良好,检出限为0.10 μg/g,定量限为0.35 μg/g。环氧乙烷测定结果的相对标准偏差为1.73%(n=6),平均加标回收率为94.3%~99.3%。该方法操作简便,精密度、准确度较高,适用于医疗防护用品中环氧乙烷的测定。
医疗保健产品灭菌环氧乙烷残留测定 测试谱图:



Determination of Sterilized Ethylene Oxide Residues in Healthcare Products
Sterilization Ethylene Oxide Residue Determination of Healthcare Products Details:
Name: Packed Column
Statio
Granularity: 60-80 mesh
Specifications: 1-2m* inner diameter 2-3mm
Model: Porapak Qs
application:
GB 18281.2-2000 Sterilization of health care products - Biological indicators - Part 2: Biological indicators for sterilization of ethylene oxide
GB 18281.2-2015 Sterilization of Healthcare Products - Ethylene Oxide - Part 1: Requirements for the Development, Validation and Routine Co
GB 18281.2-2015 Sterilization of Health Care Products Biological Indicators Part 2: Biological Indicators for Ethylene Oxide Sterilization
Haohan Chromatography (Shandong) Application Technology Development Co., Ltd. has established a gas chromatography method to detect the residual amount of ethylene oxide in medical protective equipment such as masks and protective clothing, and provide data support for manufacturers to improve the sterilization process. Gas chromatography adopts headspace injection and FID detector. The mass co
Determination of Sterilized Ethylene Oxide Residues in Healthcare Products Test Spectrum:
如果您觉得“PQ/Porapak Q填充柱 医疗保健产品灭菌环氧乙烷残留测定”描述资料不够齐全,请联系我们获取详细资料。(联系时请告诉我从给览网看到的,我们将给您最大优惠!)
本页链接:http://www.geilan.com/com_haohansepu/sell/itemid-9264790.html
已经有1234位访客查看了本页.
医疗保健产品灭菌环氧乙烷残留测定 详细信息:
名称:填充柱
固定相:高分子小球
粒度:60-80目
规格:1-2m*内径2-3mm
型号:Porapak Qs
应用:
GB 18281.2-2000 医疗保健产品灭菌 生物指示物 第2部分:环氧乙烷灭菌用生物指示物
GB 18281.2-2015医疗保健产品灭菌 环氧乙烷 第1部分:医疗器械灭菌过程的开发、确认和常规控制的要求
GB 18281.2-2015医疗保健产品灭菌 生物指示物 第2部分:环氧乙烷灭菌用生物指示物
GB 18281.2-2015医疗保健产品灭菌 生物指示物 第2部分:环氧乙烷灭菌用生物指示物
浩瀚色谱(山东)应用技术开发有限公司建立气相色谱法检测口罩、防护服等医疗防护用品中环氧乙烷的残留量,为生产厂家改进灭菌工艺提供数据支持。气相色谱法采用顶空进样、FID检测器,环氧乙烷的质量浓度在0.5~8 μg/mL范围内与色谱峰面积线性关系良好,检出限为0.10 μg/g,定量限为0.35 μg/g。环氧乙烷测定结果的相对标准偏差为1.73%(n=6),平均加标回收率为94.3%~99.3%。该方法操作简便,精密度、准确度较高,适用于医疗防护用品中环氧乙烷的测定。
医疗保健产品灭菌环氧乙烷残留测定 测试谱图:




Determination of Sterilized Ethylene Oxide Residues in Healthcare Products
Sterilization Ethylene Oxide Residue Determination of Healthcare Products Details:
Name: Packed Column
Statio
Granularity: 60-80 mesh
Specifications: 1-2m* inner diameter 2-3mm
Model: Porapak Qs
application:
GB 18281.2-2000 Sterilization of health care products - Biological indicators - Part 2: Biological indicators for sterilization of ethylene oxide
GB 18281.2-2015 Sterilization of Healthcare Products - Ethylene Oxide - Part 1: Requirements for the Development, Validation and Routine Co
GB 18281.2-2015 Sterilization of Health Care Products Biological Indicators Part 2: Biological Indicators for Ethylene Oxide Sterilization
Haohan Chromatography (Shandong) Application Technology Development Co., Ltd. has established a gas chromatography method to detect the residual amount of ethylene oxide in medical protective equipment such as masks and protective clothing, and provide data support for manufacturers to improve the sterilization process. Gas chromatography adopts headspace injection and FID detector. The mass co
Determination of Sterilized Ethylene Oxide Residues in Healthcare Products Test Spectrum:
如果您觉得“PQ/Porapak Q填充柱 医疗保健产品灭菌环氧乙烷残留测定”描述资料不够齐全,请联系我们获取详细资料。(联系时请告诉我从给览网看到的,我们将给您最大优惠!)
本页链接:http://www.geilan.com/com_haohansepu/sell/itemid-9264790.html
已经有1234位访客查看了本页.
产品咨询
相关产品推荐
相关技术文章
- icon 液相色谱柱的结构和安装
- icon 浩瀚色谱的产品成功中标航空航天企业
- icon 废水中微量甲醇和乙醇分析专用填充柱成功上市
- icon 北京大学定制的产品发货中
- icon 浩瀚色谱的产品发货到中国科学院山西煤炭化学研

高级版会员 第8年
认 证:企业信息已通过认证
主营业务:色谱仪器技术开发、技术推广技术咨询;销售:实验室设备。(依法须经批准的项目,经相关部门批准后凭许可证方可展经营活动)
- 产品目录
- 品牌分类
联系方式
联系人:王晓莹
电话:0632-5667636
手机:15562228838(微信同号)13963221227(微信同号)
邮件:wangxiaoying9@126.com
联系时,请告知信息来自给览网
















